11+ What Is The Difference Of Ionic Bond And Covalent Bond Ideas
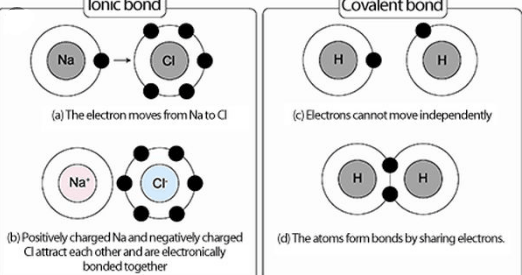
What is the difference of ionic bond and covalent bond. HowStuffWoks explains ionic and covalent bonds. From first principles we regard the covalent bond as the result of the SHARING of electron density between atoms. Ionic bonds exist between metals and non-metals. Quang cao Outperforms Any Competitive Coating. Unlike the case of molecular oxygen where the two bonded atoms share the same electronegativity carbon and hydrogen do not have the same electronegativity. And the ionic bond as the result of the transfer of electron density between atoms. See why we are the industry leading finish. The ionic bond is the bond in which an electron leaves one atom to join another while the covalent bond is the bond in which there is a sharing of an electron with two atoms. There should be unpaired electrons in order to form a covalent bond. The electronegativity difference is 12 which translates to a bond that is 30 percent ionic and 70 percent covalent and the bonds in SnCl4 are even more covalent because the electronegativity of an element increases as its oxidation number increases. Covalent bonds are formed because of sharing electrons while ionic bonds formation occurs because of transferring of electrons. In this bond the electrons are moved from one bond to another.
Metals are electron rich materials. After they link together they are connected. In an ionic bond electrons are transferred from one species to another. 7 hang An ionic bond essentially donates an electron to the other atom participating in the bond. What is the difference of ionic bond and covalent bond Covalent bonding Whereas ionic bonds involve the complete transfer of electrons between atoms covalent bonds are formed when two atoms share electrons. Molecules are the particles in covalent bonds during compound formation while in ionic bonds these are positively charged and negatively charged ions. Another example of a nonpolar covalent bond is the C-H bond found in the methane gas CH 4. Covalently bonded molecules can form a covalent structure. Start studying Difference Between Ionic and Covalent Bonds. Ionic bonds do not require molecules but covalent bonds do. See why we are the industry leading finish. Covalent bonds are molecules that are mostly gases or liquids. Learn vocabulary terms and more with flashcards games and other study tools.
 Difference Between Ionic Bond And Covalent Bond Guidance Corner
Difference Between Ionic Bond And Covalent Bond Guidance Corner
What is the difference of ionic bond and covalent bond This usually takes place between atoms of the same element or between two elements that are close to one another in the periodic table.

What is the difference of ionic bond and covalent bond. Ionic bonds involve the formation of ions and ions are held in place by electrostatic interaction. Covalent bonds involve the sharing of electrons between atoms not IONSIonic bonds can form an ionic lattice. Covalent bonds have a definite shape while ionic bonds do not have a definite shape.
An ionic bond can be differentiated from a covalent bond because of the mechanism that underlies the attraction. Covalent bonds are non-conductors while ionic bonds are conductors. Presence of Unpaired Electrons.
This is done through a force that is electrostatic. Covalent bonds form between two similar non-metals. Quang cao Outperforms Any Competitive Coating.
Only one atom donates a pair of electrons for the bond formation. C 255 and H 220the difference in electronegativity is 035. Ionic bonds are formed between metal and non-metal while covalent bonds are formed between two nonmetals.
In an ionic bond it involves atoms linking together. The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. Two atoms donate an equal number of electrons for the bond formation.
What is the difference of ionic bond and covalent bond Two atoms donate an equal number of electrons for the bond formation.
What is the difference of ionic bond and covalent bond. The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. In an ionic bond it involves atoms linking together. Ionic bonds are formed between metal and non-metal while covalent bonds are formed between two nonmetals. C 255 and H 220the difference in electronegativity is 035. Only one atom donates a pair of electrons for the bond formation. Quang cao Outperforms Any Competitive Coating. Covalent bonds form between two similar non-metals. This is done through a force that is electrostatic. Presence of Unpaired Electrons. Covalent bonds are non-conductors while ionic bonds are conductors. An ionic bond can be differentiated from a covalent bond because of the mechanism that underlies the attraction.
Covalent bonds have a definite shape while ionic bonds do not have a definite shape. Covalent bonds involve the sharing of electrons between atoms not IONSIonic bonds can form an ionic lattice. What is the difference of ionic bond and covalent bond Ionic bonds involve the formation of ions and ions are held in place by electrostatic interaction.
Indeed lately is being sought by users around us, maybe one of you personally. People now are accustomed to using the net in gadgets to view image and video information for inspiration, and according to the title of the article I will talk about about What Is The Difference Of Ionic Bond And Covalent Bond.
What is the difference of ionic bond and covalent bond. In an ionic bond it involves atoms linking together. The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. Two atoms donate an equal number of electrons for the bond formation. In an ionic bond it involves atoms linking together. The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. Two atoms donate an equal number of electrons for the bond formation.
If you are searching for What Is The Difference Of Ionic Bond And Covalent Bond you've arrived at the perfect place. We have 51 graphics about what is the difference of ionic bond and covalent bond including images, photos, photographs, backgrounds, and more. In such webpage, we additionally provide number of images out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.